中國CGT藥物研發趨勢及國內已上市CGT藥物清單
文章來源公眾號:藥事谷 作者:藥事谷
Trends in the development of cellular and gene therapy in China
中國CGT藥物研發趨勢

-
政府激勵+CDE 五年連發技術指南。
-
2017-2025 Q2共771件IND,CAR-T、干細胞、基因治療占八成。
-
NMPA共批準9款CGT產品,CAR-T產品占主導(67%),靶點集中于CD19(4款)和BCMA(3款)。
-
5款CAR-T基于單臂試驗獲批,以3個月持續緩解率為核心終點,樣本量較小(24-81例)。
-
7款附條件上市,均要求限期內(最長4年)完成確證試驗+終身隨訪。
-
CDE提出ATMP專屬性分類,推行“一企一策、研審聯動”的個案審評。
Supported by governmental incentives and a reform-oriented regulatory framework, China has become an increasingly prominent player in the global cellular and gene therapy (CGT) field.
在政府激勵和支持改革導向的監管框架雙重推動下,中國已日益成為全球細胞與基因治療(CGT)領域的重要參與者。
CGT產品的臨床試驗申請(IND)
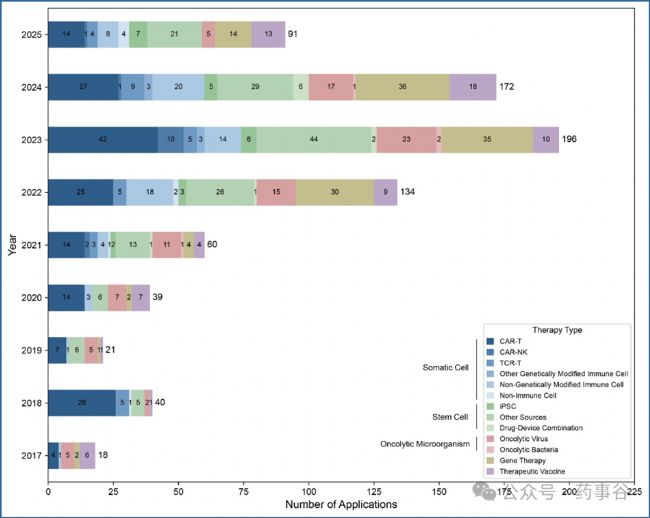
Supplementary Figure 1 | Trends in IND applications for CGT products in China (2017–2025 Q2). 補充圖1 | 中國CGT產品新藥臨床試驗申請(IND)趨勢(2017–2025年第二季度)This figure presents the distribution of IND applications for CGT products received by the CDE from 2017 to 2025 Q2. The applications are categorized into five major therapy groups: somatic cell therapy (including CAR-T, CAR-NK, TCR-T, other genetically modified immune cell, non-genetically modified immune cell and non-immune cell); stem cell therapy (including iPSCs, other stem cell sources and drug-device combination products); oncolytic microorganism (including oncolytic viruses and oncolytic bacteria); gene therapy; therapeutic vaccine.
本圖展示2017年至2025年第二季度期間,國家藥監局藥品審評中心(CDE)受理的CGT產品IND申請分布情況。申請按五大治療類別分類:體細胞治療(含CAR-T、CAR-NK、TCR-T、其他基因修飾免疫細胞、非基因修飾免疫細胞及非免疫細胞療法);干細胞治療(含誘導多能干細胞[iPSCs]、其他干細胞來源及藥物-器械組合產品);溶瘤微生物(含溶瘤病毒與溶瘤細菌);基因治療;治療性疫苗。
The analysis here mapped 765 INDs and 6 supplemental applications, for a total of 771 applications. The 6 supplemental applications were for progression of clinical trials to subsequent phases (e.g., from phase II to phase III). Under previous regulatory requirements, separate submissions were required for such progressions.
本次分析共納入765項IND申請及6項補充申請,總計771項。其中6項補充申請為推進臨床試驗進入后續階段(如II期至III期)所提交 — 按既往監管要求,此類進展需單獨提交申請。
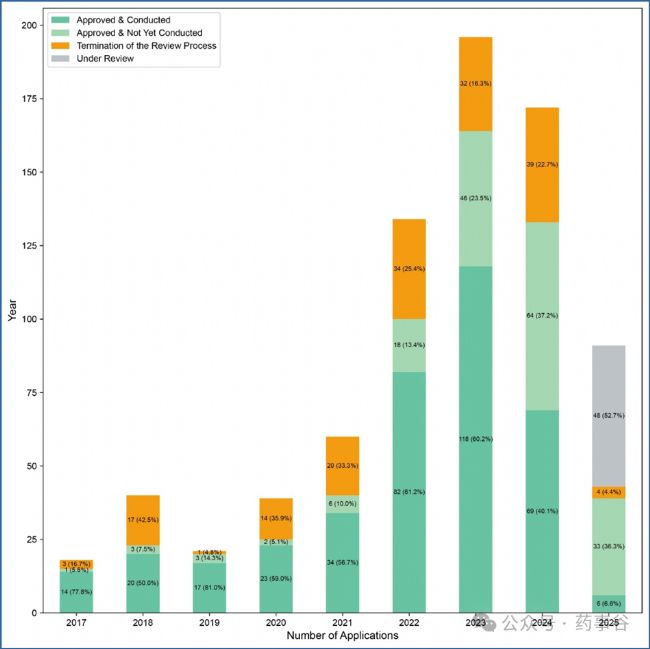
Supplementary Figure 2 | Trends in IND review conclusions and clinical trial initiation (2017–2025 Q2). 補充圖2 | IND審評結論與臨床試驗啟動趨勢(2017–2025年第二季度)IND terminations may include voluntary withdrawals by applicants or withdrawals suggested by the CDE due to a refusal to approve. The analysis here mapped 765 INDs and 6 supplemental applications, for a total of 771 applications.
本圖分析了2017年至2025年第二季度期間,國家藥監局藥品審評中心(CDE)對765項新藥臨床試驗申請(IND)及6項補充申請的審評結論(總計771項)。其中,IND終止可能包含以下兩種情況:(1)申請人主動撤回(如因研發策略調整或數據不足);(2)CDE建議撤回(因審評未通過而拒絕批準)。
By the second quarter of 2025, the CDE had reviewed a total of 765 CGT investigational new drug (IND) applications, with CAR-T, stem cell and gene therapy products comprising the majority (Supplementary Fig. 1). Of these, 553 INDs were approved for clinical trials.
截至2025年第二季度,CDE共受理了765件CGT產品的臨床試驗申請(IND)。其中,CAR-T、干細胞和基因治療產品占主要部分(補充圖1)。在這765件申請中,553件已獲準開展臨床試驗。(編者注:獲批率為72.3%,并不高!)
Common reasons for non-approval of INDs included an insufficient scientific rationale, such as an unclear mechanism of action or the absence of demonstrated clinical need in the target population; the use of illegal or unethical starting materials; inadequate pharmaceutical or nonclinical data to support the conduct of clinical trials; safety concerns identified in investigator-initiated trials; and proposed indications or dosing regimens that were off-label relative to approved product labeling in combination therapy applications.
未獲批準的主要原因包括:
-
科學依據不足,例如作用機制不明確或尚未證明目標人群存在未滿足的臨床需求;
-
起始材料來源非法或不符合倫理;
-
藥學或非臨床數據不足以支持開展臨床試驗;
-
研究者發起的臨床試驗中已發現安全性問題;
-
以及聯合用藥方案中,擬定適應癥或給藥劑量超出已獲批說明書的范圍。
Supplementary Fig. 2 shows a declining trend in IND terminations, reflecting enhanced data quality and strengthened CDE guidance and pre-IND engagement, all of which have contributed to a more supportive environment for CGT development.
補充圖2顯示,IND被終止的數量呈下降趨勢,反映出數據質量持續提升、CDE指導原則不斷完善以及pre-IND溝通交流日益充分,共同為CGT開發營造了更加有利的環境。(編者注:美國Pre-IND僅有一次機會,而在國內,Pre-IND沒有次數限制,這給完善研究和申報資料提供了充分的“準備時間”,對提高IND申報成功率的作用自然是顯而易見的。而另一個不爭的事實是,美國申報IND的效率明顯高于國內,同時存在在美國成功拿到IND,而國內沒有獲批的情況。)
Clinical trial landscape and characteristics of investigational CGT products.
CGT產品臨床試驗概覽及特征
The development stage for CGT products in clinical trials in China is shown in Fig. 1.Among the various CGT modalities, CAR-T products are the most advanced overall, with a total of 72 products in exploratory trials, 15 in confirmatory trials, 7 under new drug application (NDA) review and 9 approved for marketing (Supplementary Table2). An additional 45 INDs have been approved for products for which clinical trials have not yet been initiated.
圖1展示了中國境內CGT產品臨床試驗所處的階段。從各類CGT技術路徑來看,CAR-T產品整體進展最快:共有72個產品處于探索性臨床試驗階段,15個處于確證性臨床試驗階段,7個正在新藥上市申請(NDA)審評中,9個已獲批上市(補充表2,公眾號回復“CGT”可獲取論文和上市CGT產品統計表)。此外,另有45個產品的IND申請雖已獲批,但臨床試驗尚未啟動。
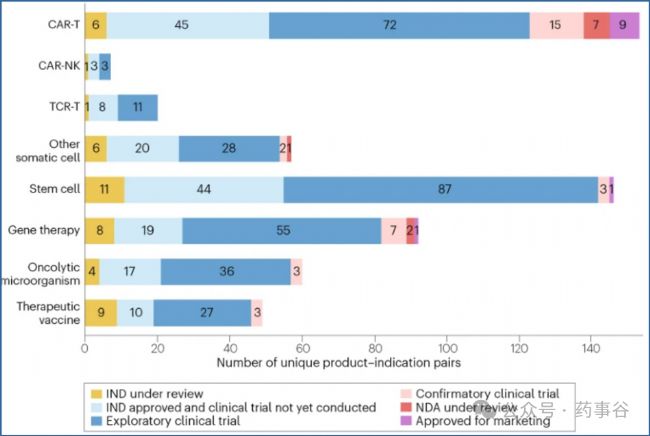
Fig. 1 | Clinical development status for investigational cell and gene therapy products in China. 圖1 |中國細胞與基因治療產品在研項目的臨床開發階段分布
Distribution of investigational cell and gene therapy products across various clinical development stages: investigational new drug (IND) application under review, IND approved and clinical trial not yet conducted, exploratory clinical trial, confirmatory clinical trial, new drug application (NDA) under review and approved for marketing. Data are categorized by therapeutic type: CAR-T, CAR-NK, TCR-T, other somatic cell, stem cell, gene therapy, oncolytic microorganism and therapeutic vaccine. The x-axis represents the total number of indications across all products.
圖中展示了在研細胞與基因治療產品在不同臨床開發階段的分布情況,包括:IND申請審評中、IND已獲批但尚未開展臨床試驗、探索性臨床試驗、確證性臨床試驗、NDA審評中以及已獲批上市。數據按治療類型分類:CAR-T、CAR-NK、TCR-T、其他體細胞產品、干細胞產品、基因治療、溶瘤微生物及治療性疫苗。橫軸表示所有產品所對應的適應癥總數。
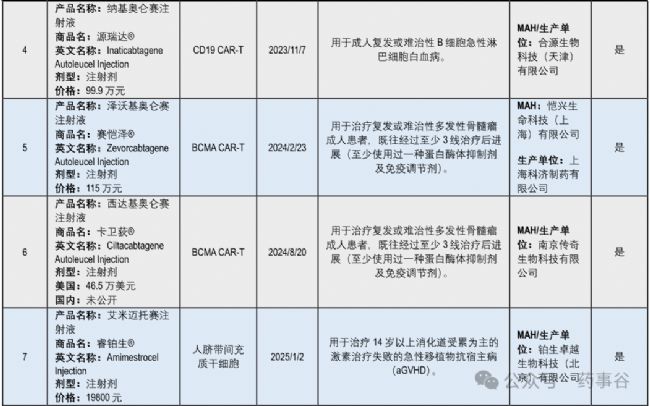
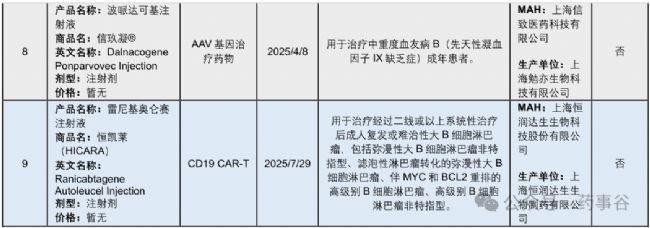
The second most active area of clinical trial activity is for stem cell therapies, with 44 IND-approved products pending trial initiation, 87 in exploratory trials, 3 in confirmatory trials and 1 approved product (amimestrocel injection for graft-versus-host disease). The gene therapy area also has substantial activity, with 55 products in exploratory trials, 7 in confirmatory trials, 2 under NDA review, and 1 product approved for marketing recently (dalnacogene ponparvovec for haemophilia type B). Most other CGT modalities remain in early stages of development, but the data in Fig.1 illustrate the diversifying CGT landscape in China.
臨床試驗活躍度位居第二的是干細胞療法:已有44個IND 獲批產品尚未啟動試驗,87個處于探索性臨床試驗階段,3個處于確證性臨床試驗階段,并有1個產品已獲批上市(用于移植物抗宿主病的amimestrocel注射液)。基因治療領域同樣活躍:55個產品處于探索性臨床試驗階段,7個處于確證性臨床試驗階段,2個正在進行NDA審評,并已有1個產品近期獲批上市(用于治療B型血友病的dalnacogene ponparvovec)。其余大多數CGT技術路徑仍處在早期開發階段,但圖1的數據清晰表明,中國的CGT研發格局正日益多元化。
The indications and targets for the three main therapy categories — somatic cell therapies (including CAR-T cell therapies, CAR-natural killer (NK) cell therapies and T-cell receptor (TCR) T cell therapies), stem cell therapies and gene therapies — were analysed further (Fig. 2).
圖2進一步分析了三大主要療法類別—體細胞療法(包括CAR-T細胞治療、CAR-自然殺傷(NK)細胞治療和T細胞受體(TCR)T細胞治療)、干細胞療法以及基因治療—所針對的適應癥與靶點。
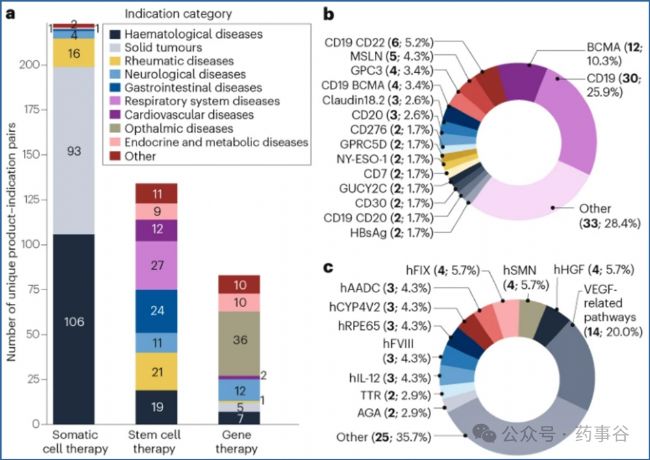
Fig. 2 | Distribution of therapeutic indications and targets for investigational cell and gene therapy products in China. 圖2|中國細胞與基因治療在研產品適應癥與靶點分布 a, The indication analysis includes somatic cell therapies, stem cell therapies and gene therapies. The indication count follows the same criteria as used in Fig. 1. b, Target analysis of somatic cell therapies. c, Target analysis of gene therapies. Targets are counted based on the number of products with approved investigational new drug applications. Products targeting dual or multiple targets are counted as a single combination and are not listed separately.
a,適應癥分析覆蓋體細胞治療(含CAR-T、CAR-NK、TCR-T等)、干細胞治療及基因治療;適應癥計數方式與圖1保持一致。
b,體細胞治療的靶點分析。
c,基因治療的靶點分析。
靶點統計以已獲批IND的產品數量為基準;針對雙重或多重靶點的產品按單一組合計數,不再拆分列出。
Somatic cell therapies are primarily focused on cancers (both blood cancers and solid tumours). Blood cancer targets expressed on B-cells such as CD19 and BCMA are the most popular (Fig. 2b), and multi-target approaches are being explored. Encouraged by a pioneering clinical trial showing the potential of B-cell depletion with CD19-targeted CAR-T cell therapy to lead to treatment remission for patients with systemic lupus erythematosus (SLE), such therapies are now also being investigated for other non-oncological indications such as myasthenia gravis and systemic sclerosis.
體細胞治療主要集中于腫瘤領域,涵蓋血液腫瘤和實體瘤。其中,以B細胞表面抗原CD19和BCMA為靶點的血液腫瘤治療最為熱門(圖2b),且多靶點策略正在積極探索。受一項具有開創性的臨床試驗啟發——該試驗顯示,靶向CD19的CAR-T細胞療法可通過耗竭B細胞,使系統性紅斑狼瘡(SLE)患者獲得病情緩解——目前此類療法也正被拓展至其他非腫瘤適應癥,如重癥肌無力和系統性硬化癥的研究中。
Stem cell therapies in clinical trials have a broad spectrum of indications, with applications varying according to the specific functional properties of different stem cell types. Gene therapies are largely focused on rare genetic diseases, including haemophilia types A and B, inherited retinal diseases, spinal muscular atrophy and inborn errors of metabolism. Gene therapies for eye diseases targeting VEGF-related pathways are particularly popular, with 14 IND-approved products (Fig. 2c).
臨床試驗中的干細胞治療適應癥范圍廣泛,不同干細胞類型因其特有的功能屬性而被應用于不同疾病領域。基因治療則主要集中在罕見遺傳病,包括A型和B型血友病、遺傳性視網膜疾病、脊髓性肌萎縮癥以及先天性代謝缺陷。其中,針對眼部疾病、靶向VEGF相關通路的基因治療尤為熱門,已有14個產品獲得IND批準(圖2c)。
Approved CGT products in China and post-marketing management.
中國已獲批的CGT產品及上市后管理
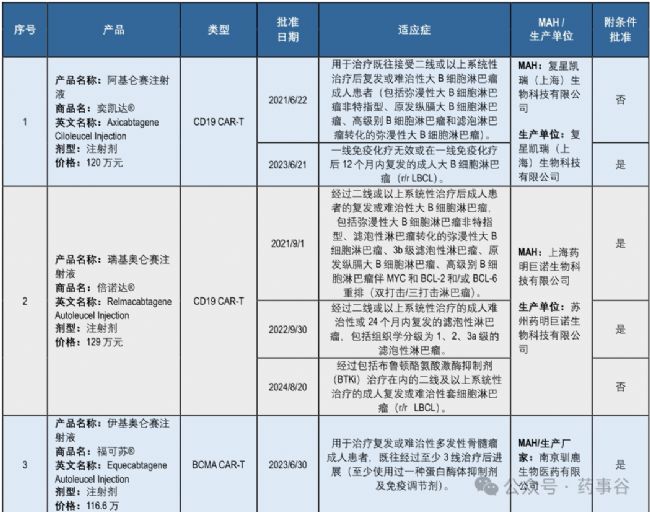
So far, the NMPA has approved six CAR-T cell therapies for nine indications (including seven under conditional approval and two with full approval), along with one conditionally approved stem cell therapy and one fully approved gene therapy, each for a single indication (Supplementary Table 2).
截至目前,國家藥監局已批準6款CAR-T細胞治療產品,共覆蓋9個適應癥(其中7個為附條件批準,2個為完全批準);此外,還批準了1款附條件上市的干細胞療法和1款完全批準的基因療法,二者各對應1個適應癥(補充表2)(文末見編者最新統計更新)。
Five CAR-T cell therapy products have been approved in China based on single-arm clinical trials. These trials used tumor response at no less than three months after CAR-T infusion as the primary endpoint, in line with the CDE’s requirement to assess the durability of response.
在中國,已有5款(注,統計截止文章)CAR-T細胞治療產品基于單臂臨床試驗獲批。這些試驗均以CAR-T回輸后至少3個月的腫瘤緩解率作為主要終點,符合CDE對療效持久性的評估要求。
All products demonstrated a favorable benefit–risk profile, with efficacy results significantly superior to existing therapies. Given the limited sample sizes in these studies, marketing authorization holders are required to conduct post-marketing studies to further verify product efficacy and safety.
所有產品均顯示出有利的獲益-風險特征,療效結果顯著優于現有治療手段。鑒于研究樣本量有限,上市許可持有人必須開展上市后研究,以進一步驗證產品的有效性和安全性。
For conditionally approved products, confirmatory clinical trials should be completed within a required period of time from the date of approval, up to a maximum of four years. Long-term follow-up as well as real-world studies are required to monitor patients receiving CAR-T therapies.
對于附條件批準的產品,應自獲批之日起在規定期限內完成確證性臨床試驗,最長期限不超過四年。同時,還需進行長期隨訪及真實世界研究,以監測接受CAR-T治療的患者。
Additionally, one CAR-T product that had already been approved overseas, axicabtagene ciloleucel, was evaluated in China through a bridging study and foreign clinical studies. The primary endpoint of the bridging trial was consistent with that of the foreign study. The acceptance of foreign studies in future applications remains subject to communication with the CDE.
此外,一款已在海外獲批的CAR-T產品—阿基侖賽(Axicabtagene Ciloleucel)—通過一項橋接試驗并結合境外臨床研究數據在中國完成了評價。該橋接試驗的主要終點與境外研究保持一致。未來申請中能否繼續接受境外研究數據,仍需與CDE進行溝通確認。
Post-marketing risk management for CAR‑T cell therapies in China is characterized by a comprehensive, lifecycle‐wide approach that rigorously safeguards patient safety and ensures therapeutic efficacy, with particular attention to the surveillance of secondary malignancies, such as T‑cell lymphomas.
中國對CAR-T細胞治療的上市后風險管理采取覆蓋全生命周期的綜合策略,以切實保障患者安全并確保療效,尤其重視繼發惡性腫瘤(如T細胞淋巴瘤)的監測。
Although international investigations have detected CAR transgenes in malignant T‑cell clones in a limited number of cases, no instances of secondary T‑cell lymphoma have been reported in China.
盡管國際研究曾在極少數病例的惡性T細胞克隆中檢出CAR轉基因,但國內迄今尚未報告繼發T細胞淋巴瘤案例。
In practice, risk control measures are embedded throughout the development and marketing process. Clinical trial documentation emphasizes vigilant monitoring for secondary malignancy risks; product labels explicitly warn of these risks and require lifelong patient follow‑up; and, upon detection of any malignant events, standardized protocols for the timely collection and molecular analysis of pre‑and post‑treatment biological samples are activated.
在實踐中,風險控制措施貫穿研發與上市全過程:
-
臨床試驗文件強調對繼發惡性腫瘤風險的嚴密監測;
-
產品說明書明確警示該風險并要求對患者進行終身隨訪;
-
一旦監測到任何惡性事件,將立即啟動標準化流程,及時收集并開展治療前后生物樣本的分子分析。
Regulatory outlook for CGT in China
中國CGT監管展望
Looking ahead, China’s approach is expected to emphasize unified deployment while following the principles of “early involvement, one enterprise one policy, whole-process guidance, and research–review linkage“.
The CDE may adopt a case-by-case review approach, offering tailored strategies for different products based on their specific risks, benefits and manufacturing complexities.
CDE可能采取逐案審評模式,依據不同產品在風險、獲益及生產工藝復雜度等方面的差異,制定個性化策略。As the CGT field evolves rapidly, China is set to continually refine its regulatory framework to support innovation.
隨著CGT 領域日新月異的發展,中國將持續優化監管框架,以更好地支持創新。中國批準的CGT藥物匯總